helium electron configuration|2.4 Electron Configurations : Manila 119 rows — Mar 23, 2023 — Find the electron configuration of helium (He) and . Watch Catryona Lei porn videos for free, here on Pornhub.com. Discover the growing collection of high quality Most Relevant XXX movies and clips. No other sex tube is more popular and features more Catryona Lei scenes than Pornhub! Browse through our impressive selection of porn videos in HD quality on any device you own.
PH0 · How to Write the Electron Configuration for Helium
PH1 · Helium Electron Configuration – Bohr and Aufbau Principle
PH2 · Electron Configuration Chart of All Elements (Full Chart)
PH3 · 4.2: Quantum
PH4 · 3.1: Electron Configurations
PH5 · 2.7: Electron Configurations
PH6 · 2.4 Electron Configurations
PH7 · 2.2: Electron Configurations
PH8 · 1.5 Atomic Structure and Electron Configuration
Nitrogen is a chemical element; it has symbol N and atomic number 7. . In the ground state, they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 z. It, therefore, has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired.
helium electron configuration*******Ago 22, 2024 — Chemists use an electronic configuration to represent the organization of electrons in shells and subshells in an atom. An electron configuration simply lists the shell and subshell labels, with a right superscript giving the number of electrons in that subshell.119 rows — Mar 23, 2023 — Find the electron configuration of helium (He) and .Learn how to write the electron configuration for helium, a noble gas with two electrons in the 1s orbital. See a video, a chart and a step-by-step tutorial for writing electron configurations.Ene 29, 2024 — Learn how to arrange electrons in the helium atom's orbitals using the Bohr model and the Aufbau principle. Find out the electron configuration, group, period, valency, and .
helium electron configuration 2.4 Electron Configurations This structure is called an electron configuration and is unique to hydrogen. Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells contain one s orbital .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of .2.4 Electron Configurations Learn how to write the electron configuration of an atom using the periodic table and the rules of orbital occupation. See examples of helium, hydrogen, oxygen, nitrogen, and other elements.
For the representative elements (columns 1, 2, and 13-18 of the Periodic Table), the core electrons are all electrons with an n-value lower than the maximum n-value in the electron .
Hul 20, 2022 — Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is .Hul 20, 2022 — Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”). Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; and f, 14 (Table .Hun 26, 2016 — Helium, #"He"#, has an atomic number equal to #2#, which means that it has #2# protons in its nucleus.. A neutral helium atom will thus have #2# electrons surrounding its nucleus. This means that the electron .helium electron configurationHun 27, 2024 — Let us look at the electron configuration for helium, which is 1 s 2 1{\rm s}^2 1 s 2. The first integer, 1 1 1, indicates the primary energy level. In general, its values can be between 1 1 1 to n n n, where n n n is the value of the outermost shell containing an electron.
A helium atom is an atom of the chemical element helium.Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with two neutrons, depending on the isotope, held together by the strong force.Unlike for hydrogen, a closed-form solution to the Schrödinger equation for the helium atom has not been found.
Nob 14, 2013 — A step-by-step description of how to write the electron configuration for Helium (He). In order to write the He electron configuration we first need to know.
Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. . Helium (He) – 1s 2; Neon (Ne) – [He]2s 2 2p 6; Argon (Ar) – [Ne]3s 2 3p 6; Krypton (Kr .
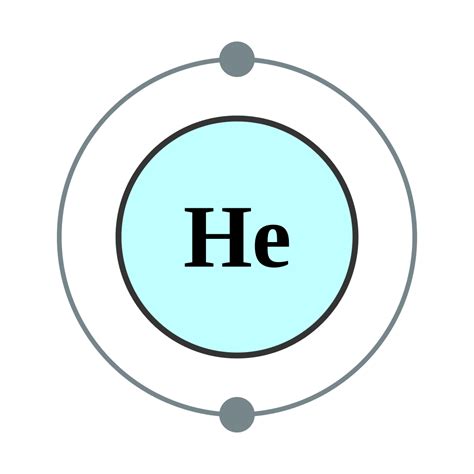
Electron configuration of Helium is 1s2. Possible oxidation states are 0. Helium is small and extremely light, and is the least reactive of all elements; it does not react with any other elements or ions, so there are no helium-bearing minerals in nature. High-energy electron-scattering experiments show its charge to decrease exponentially from .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and 2p subshells .Nob 26, 2022 — The electronic configuration notation of He is [He] 1s 2. Helium unabbreviated electron configuration. The unabbreviated electronic configuration of He is 1s 2. As it contains only 2 electrons so its unabbreviated electron configuration is the same as the electronic configuration. Ground state Helium electron configuration. The ground state .Ene 15, 2024 — This structure is called an electron configuration and is unique to hydrogen. Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells contain one s orbital which can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”).Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating .The single electron is assigned to the 1s sublevel, the lowest-energy sublevel in the lowest-energy level. Therefore, the electron configuration of hydrogen is written: For helium (atomic number 2), which has two electrons, the electron configuration is: He: 1s 2. Two electrons completely fill the first energy level.
Hun 30, 2023 — Helium is the second most abundant element in the universe, next to hydrogen. Helium is colorless, odorless, and tasteless. It has a very low boiling point, and is monatomic. . Electron Configuration : 1s 2: Number of Isotopes: 7 (2 liquid) Electron Affinity: 0 kJ/mol: First Ionization Energy: 2372.3 kJ/mol:Here is the electron configuration for Helium: 1 s 2. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. In this case, there are two electrons in an s orbital with the principle energy level of one.
Ene 3, 2021 — Helium Electron Configuration: In the periodic table, the second chemical element is Helium whose symbol is “He” and its atomic number is 2. Helium is the chemical element that is tasteless, colorless, odorless, inert, non-toxic, monoatomic gas and it lies in the noble gas group category. This element has the lowest boiling point.

Ago 14, 2020 — The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a condensed .
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point . Once it has grabbed a couple of electrons, a helium atom has been born. This decay process for uranium is incredibly slow; the time it takes a given quantity of uranium to halve, its so-called half-life, is comparable to the age of .
Dis 14, 2021 — To write the orbital diagram for the Helium (He) first we need to write the electron configuration for just He. To do that we need to find the number of ele.
droskop droskop.com
helium electron configuration|2.4 Electron Configurations